Abstract
Background and Objectives:
Inherited Dysfibrinogenemia is a rare functional fibrinogen disorder in which the fibrinogen protein is present but with a reduced function. Fibrinogen is a 340-kDa glycoprotein that is encoded by three genes namely: Fibrinogen Bb (FGB), Aa (FGA), and g (FGG). The disorder is characterized by a wide spectrum of clinical phenotypes, ranging from asymptomatic to mild- to-severe bleeding or thrombotic manifestations and recurrent miscarriages. The mode of inheritance is mostly autosomal dominant manner and frequently as a result of a point mutation in FGA (Arg35) and FGG (Arg301).
The laboratory diagnosis is based on discrepancy between fibrinogen antigen (detected by immunoassay or by immuno-turbidimetric assay) and functional assay (detected by Clauss method or other clot-based assays). The disorder is often associated with prolonged activated partial thromboplastin time (APTT), prothrombin time (PT) and thrombin time (TT).Fibrinogen activity is reduced by Clauss method while the antigen assay remains normal.
The management is directed towards prevention of bleeding with prophylactic fibrinogen concentrates or cryoprecipitate prior to invasive procedures, surgeries or delivery.
Dysfibrinogenemia is a rare disorder yet it is very prevalent in Qatar as a result of high rate of consanguineous marriages. The aim of our study is to describe the clinical phenotype in relation to genotype in this cohort.
Methods
We conducted a retrospective analysis of 23 patients with Inherited Dysfibrinogenemia reported by our center from 2015 to 2020 . Patients with a positive family of history fibrinogen disorder and abnormal coagulation screen, low functional fibrinogen assay (by Clauss method) or normal antigen level by turbidimetry were included. Whole exome sequencing (WES) was performed on the proband case which detected a likely pathogenic mutation that was tested on subsequent cases.
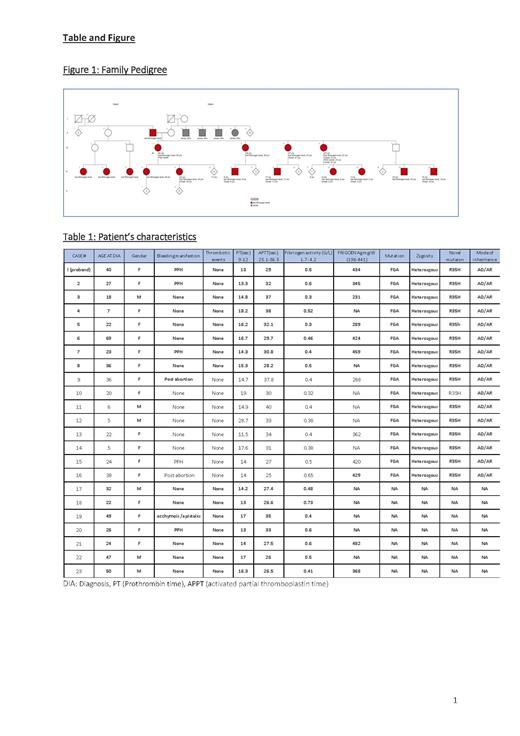
We diagnosed our patients with Inherited Dysfibrinogenemia based on both coagulation-based assays and molecular tests. Probable Inherited Dysfibrinogenemia was considered in patients where the molecular test or antigen assay were not performed. To assess the clinical phenotype, data was collected that included; age at diagnosis, gender, bleeding and thrombotic events as well as coagulation screening. (Table 1)
Results
23 patients who were described in this cohort belong to the same tribe. 74% (17 o/23) were female and only 41% (7/17) reported an obstetric bleeding (postpartum or post abortion) and one reported mild bleeding that occurred in the postmenopausal period and no previous bleeding (case#19). The median age of diagnosis was 28.8 years (5-69) for the females. All male cases in the cohort were detected either during routine screening or prior to surgery with no previous history of bleeding. No thrombotic events were observed in this cohort.
Genetic Analysis
Following proper genetic counseling and informed consent, whole exome sequencing analysis (WES) was performed on the index case which included testing of the fibrinogen genes FGA, FGB and FGG. WES revealed a likely pathogenic mutation in the FGA gene (p. Arg35His (R35H) (CGT>CAT): c.104 G>A in exon 2)-Located within the cleavage site of fibrinopeptide A by thrombin (The UniProt Consortium, 2017), which is a mutational hotspot.
This result is likely consistent with the diagnosis of Dysfibrinogenemia.
Conclusion
The FGA R35H mutation is considered a probable recurrent variant in a large tribe in the Qatari population and is associated with late onset mild bleeding manifestations in minority of cases . Despite the fact that the reported tribe is highly consanguineous, the R35H mutation behaved in an autosomal dominant manner rather than recessive in this cohort.Further studies to assess phenotype - genotype correlation of Dysfibrinogenemia is warranted.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal